IB Chemistry Topic 3: Periodicity Notes
3.1 The Periodic Table
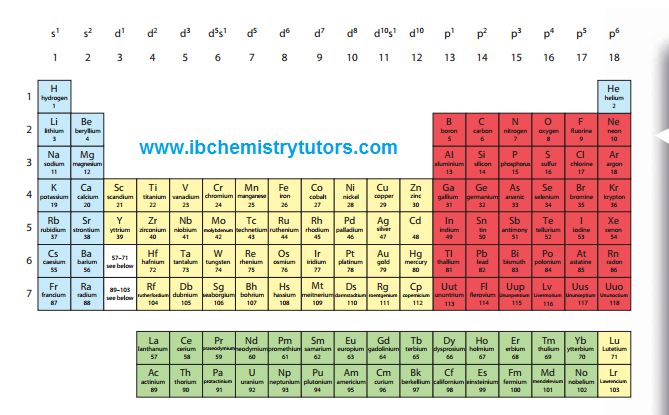 The Periodic Table arranges elements according to increasing atomic number or number of protons.
The Periodic Table arranges elements according to increasing atomic number or number of protons.- The horizontal rows are called periods and vertical columns called groups.
- Elements in the same group have the same number of outer electrons and have similar chemical properties.
- The elements in the same period are arranged according to increasing atomic number, and have outer electrons in the same energy level.
- The position of an element is related to the electron arrangement of its atoms. Magnesium, for example, is in Period 3, as it has three occupied energy levels and in Group 2, as there are two electrons in its outer energy level. Boron is in Period 2, as it has two occupied energy levels and in Group 3 as there are three electrons in its outer energy level.
- The chemical and physical properties of elements arranged in order of increasing atomic numbers vary periodically. Periodicity is the regular repetition of properties of elements arising from patterns in their element arrangement.
- The chemical properties of elements are generally due to the number of electrons in the outer energy level of their atoms.
- Metals are found on the left of the table and non-metals on the right of the Periodic Table.
- The transition metals are in the large d-block elements in the middle of the Periodic Table from Sc to Zn etc. Sc and Zn are not transition metals because they do not form ions with incomplete d sub-levels.
Topic 3: Periodicity
3.2 Physical properties
- The first ionization energy (IE) of an element is the minimum energy required to form a mole of singly charged positive ions (M + ) by removing an electron from each atom (M) in the gaseous state: M (g) ® M + (g) + e– (units: kJ mol–1).
- Electronegativity is a measure of how strongly the atom attracts the electrons in a covalent bond.
- As a group is descended and the number of occupied energy levels increases the atomic radii and ionic radii increase.
- As a group is descended both first ionization energies and electronegativities decrease due to the increased distance between the nucleus and the outer energy level, which reduces the force of attraction between the nucleus and the outer electrons.
- The melting points decrease down Group 1 as there is a decrease in the strength of the metallic bonding. The increase in ionic radii reduces the force of the attraction between the M + ions and the delocalised electrons.
- The melting points increase down Group 7, as there is an increase in the strength of van der Waals’ forces with increasing number of electrons.
- Across Period 3 the atomic radii decrease and the first ionization energies and electronegativities increase as the nuclear charge increases and electrons are added to the same outer shell. The attraction between the outer electrons and nucleus increases.
- Patterns in ionic radii in Period 3 are more complex:
- Positive ions are smaller than their parent atoms, as the formation of positive ions involves the loss of the outer shell.
- Negative ions are larger than their parent atoms, as their formation involves the addition of electrons into the outer shell. The increased electron repulsion between the electrons in the outer energy level increases the radius of the outer shell.
- The ionic radii decrease from Groups 1 to 4 for the positive ions. The ions Na +, Mg2 +,
Al3 + and Si4 + are isoelectronic and have the same electron arrangement 2, 8. The decrease in ionic radius is due to the increase in nuclear charge with atomic number across the Period, which increases the attraction between the nucleus and the outer electrons. - The ionic radii decrease from Groups 4 to 7 for the negative ions. The ions Si4–, P3–, S2– and Cl– are isoelectronic and have the same electron arrangement 2, 8, 8. The decrease in ionic radius is due to the increase in nuclear charge across the Period.
- Positive ions are smaller than the negative ions, as the former have only two occupied electron shells and the latter have three.
- The noble gases are not assigned electronegativities as they do not readily form bonds with other elements.
- The electronegativities of diagonal elements remain approximately the same as both the group and period number increase. Boron and aluminium, for example, both have electronegativities of 1.6.
- The electronegativity of H is the same as that of P.
Topic 3: Periodicity
3.3 Chemical properties
Alkali metals
- All the metals are too reactive to be found in nature.
- They generally donate electrons and act as reducing agents: M ® M + + e–.
- Reactivity increases down the Group with the decrease in ionization energies.
- Their ability to conduct electricity is also due to the mobility of their outer electron.
- The alkali metals react with water to produce hydrogen and the metal hydroxide. The resulting solution is alkaline owing to the presence of hydroxide ions:
2M (s) + 2H2O (l) ® 2MOH (aq) + H2 (g)
Halogens
- The halogens are diatomic non-polar molecules.
- They generally accept electrons and act as oxidizing agents: X2 + 2e– ® 2X–.
- Reactivity decreases down the Group, as the atom gets larger and attraction for extra electrons decreases.
- The more reactive halogens, X2, displace the less reactive halogens, Y, from their compounds:
X2 + 2Y– ® Y2 + 2X–
E.g. the more reactive Cl displaces Br: Cl2 + 2Br– ® Br2 + 2Cl–
- The halogens react with the Group 1 metals to form ionic halides:
2M + X2 ® 2 MX.
The most vigorous reaction occurs between the elements which are furthest apart in the Periodic Table.
Period 3 oxides
Pages: 1 2








