IB Chemistry Topic 1: Stoichiometric relationships. These notes are written by expert IB Chemistry tutors.
1.1 The mole concept and Avogadro’s constant
· 
 The amount of substance (n) is measured in moles (mol).
The amount of substance (n) is measured in moles (mol).
· The mole concept applies to all species: atoms, molecules, ions, electrons, formula units.
· 1 mol contains the same number of chemical species as there are atoms in exactly 12 g of the isotope carbon-12.
· 1 mol of any substance contains 6.02 ´ 1023 species.
· 6.02 ´ 1023 mol–1 is called Avogadro’s constant (L). It has units as it is the number of particles per mole.
· The relative atomic mass (Ar) of an element is the average mass of an atom according to relative abundances of its isotopes, on a scale where the mass of one atom of Carbon (6, 12) is 12 exactly. It has no units.
· The relative molecular mass (Mr) is the sum of the relative atomic masses of the atoms in the molecular formula.
· The relative formula mass of an ionic compound is the sum of the relative atomic masses of the ions in the formula.
· The molar mass (M) is the relative mass expressed in g and has units of g mol–1.
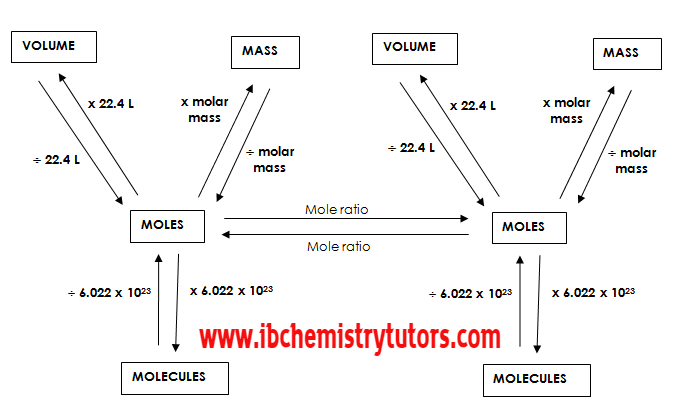
· Number of mol = mass / molar mass
· Number of particles = number of mol ´ Avogadro’s constant
· n = m / M
· N = nL
Topic 1: Stoichiometric relationships
1.2 Formulas
· The molecular formula shows the number of atoms of each element present in a molecule.
· The empirical formula gives the ratio of the atoms of different elements in a compound. It is the molecular formula expressed as its simplest ratio. The molecular formula is a whole-number multiple of the empirical formula.
· The empirical formula of a compound containing the elements X, Y and Z can be determined by completing the following table:
| Mass/ g or % of X | Mass/ g or % of Y | Mass/ g or % of Z | |
| Mass/ g | mX | mY | mZ |
| n/mol | = mX/MX | = mX/MX | = mX/MX |
| Simples ratio (divide by smallest amount in previous row) |
1.3 Chemical equations
· The coefficients in a chemical equation describe the relative amounts of reactants and products.
| For a reaction pX + qY® rZ where p, q and r are coefficients: | nX/nY = p/q or nZ/nZ = p/r etc |
· State symbols indicate the state of a substance: (s) solid, (l) liquid, (g) gas and (aq) aqueous solution or dissolved in water.
1.4 Mass and gaseous volume relationships in chemical reactions
· The limiting reactant determines the theoretical yield of product. The other reagents are in excess.
· The theoretical yield is the mass or amount of product produced according to the chemical equation assuming 100% reaction of the limiting reagent.
· Percentage yield = (experimental yield/ theoretical yield) ´ 100%
· The kelvin is the SI unit of temperature: T (K) = T (°C) + 273
· Units of volume: 1 dm3 = 1 litre = 1 ´ 10–3 m3 = 1 ´ 103 cm3 = 1000 ml
· Temperature (in K) is a measure of the average kinetic energy of the particles. Particles have minimum kinetic energy at absolute zero (0 K).
· As kinetic energy = ½mv2 and all gases have the same kinetic energy at the same temperature, particles with smaller mass move faster.
· Avogadro’s hypothesis states that equal volumes of different gases contain equal numbers of particles at the same temperature and pressure.
· Number of mol = volume/molar volume n = V/Vmol
· The coefficients in a chemical equation describe the relative amounts of reactants and products.
| Gay–Lussac’s law: For a reaction pX (g) + qY (g) ® rZ (g) where p, q and r are coefficients: | volX / volY = p / q or volX / volZ = p / r etc. |
· Molar volume, Vm, of any gas at STP = 22.4 dm3.
· STP for gases is standard temperature (0°C or 273 K) and pressure (1 atmosphere or 100 kPa).
1.5 Solutions
· Density = mass / volume
· ρ = m/v
· A solution is a homogeneous mixture of a liquid (the solvent) with another substance (the solute). The solute can be solid, liquid or gas but the solvent is generally a liquid.
· Concentration is the amount of solute in a known volume of solution. It can be expressed either in g dm–3 or mol dm–3. Concentration in mol dm−3 is often represented by square brackets around the substance:
| [solute] (mol dm−3) = nsolute (mol) / Vsolution (dm3) | nsolute = [solute] ´ Vsolution (dm3) nsolute = [solute] ´ Vsolution (cm3) / 1000 |
· Titration is a chemical technique in which one solution is used to analyse another solution to find its concentration or amount.
Click here to get the PDF of the notes.
Definitions involved in IB Chemistry Topic 1: Stoichiometric relationships
Amount The number of moles of a substance present in a sample.
Aqueous solution A solution with water as the solvent.
Atmosphere The unit atmosphere is used to measure pressure. 1 atm. = 101 325 Pa. It is not an SI unit.
Atmospheric pressure The pressure exerted by the atmosphere on the surface of the Earth due to the weight of the gases in the atmosphere.
Avogadro constant, L The Avogadro constant (6.02 ´ 1023) is the number of atoms in exactly 12 grams of carbon-12. It has units of mol–1.
Avogadro’s hypothesis This states that at a specified temperature and pressure, equal volumes of (ideal) gases contain equal numbers of moles of particles. There is a direct relationship between the volume of gas, V, and the amount of particles, n: V µ n.
Back titration An experimental procedure which typically consists of two consecutive acid-base titrations performed when an insoluble and slowly reacting reagent is treated with an excess of an acid or base. The excess acid or base is then titrated and neutralised with a primary standard. It can be used, for example, to analyse the amount of calcium carbonate in a shell or the amount of aspirin in a tablet.
Balanced equation A summary of a chemical reaction using chemical formulae. The total number of any of the atoms and charges is the same on the reactant and product sides of the equation.
Boiling The conversion of a liquid into a gas at constant temperature when the vapour pressure of the liquid is equal to the external pressure. It is characterised by the appearance of bubbles of vapour throughout the liquid.
Boiling point The temperature at which a liquid is converted to a gas. A liquid boils when the vapour pressure of the liquid equals the surrounding pressure.
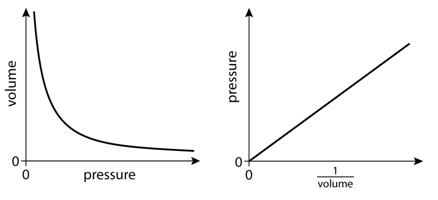
Boyle’s law This states that the pressure of a fixed mass of ideal gas is inversely proportional to the volume at constant temperature. P µ 1/V.
By-products Unwanted products of a chemical synthesis or manufacturing process.
Celsius scale (of temperature) This scale of temperature is based on a 100-degree range between the normal melting point of pure ice (0°C) and the normal boiling point of pure water (100°C).
Change of state The inter-conversions of a substance between the solid, liquid and gaseous states.
Charles’ law This states that the volume of a fixed mass of an ideal gas at constant pressure is directly proportional to its absolute temperature. V µ T.
Chemical element A substance which cannot be decomposed or broken down into simpler substances by chemical methods. All atoms of an element contain the same number of protons.
Chemical reaction A change in which a new substance or substances are formed.
Chemical symbol This consists of one or two letters used to represent each element. The first (or only) letter is always a capital letter, the second is lower case.
Coefficient (in a chemical equation) The coefficients in a chemical equation are the numbers that appear on the left of chemical formulae.
Combined gas law This relates the pressure, absolute temperature and volume, under two sets of conditions for a fixed mass of gas. P1V1/T1 = P2V2/T2.
Completion Complete consumption of at least one of the reactants in a chemical reaction. A reaction goes to completion if its limiting reactant or reagent is consumed.
Compound A substance formed by the chemical combination of two or more chemical elements in fixed proportions.
Concentrated A concentrated solution contains a relatively high concentration of solute.
Concentration This is the amount of solute in a known volume of solution. It can be expressed either in g dm–3 or mol dm–3. Concentration in mol dm–3 is often represented by square brackets around the substance: [solute] (mol dm–3) = nsolute (mol) / Vsolution (dm3)
Condensing The change of a vapour into a liquid at constant temperature. Latent heat is released to the surroundings during this process, as bonds between the particles in the liquid are formed.
Dalton’s law of partial pressures The total pressure of a mixture of ideal gases is the sum of the partial pressures of all the component gases.
Density Density is defined as mass per unit volume. Density (ρ) = mass (m) / volume (V).
Diffusion The spontaneous movement of gas or liquid particles from a region of high concentration of the particles to a region of low concentration.
Dilute A dilute solution contains a relatively low concentration of solute.
Dilution The process of adding more solvent, usually water, to a solution to decrease the concentration.
Empirical formula This gives the ratio of the atoms of different elements in a compound. It is the molecular formula expressed as its simplest ratio. The molecular formula is a whole-number multiple of the empirical formula.
Evaporation Evaporation occurs at the surface of the liquid and involves a liquid changing into a gas at a temperature below the boiling point of the liquid.
Excess A reactant is in excess when, after the reaction is complete, some of that reactant remains unreacted.
Formula mass This is the sum of the atomic masses of the elements in the formula of an ionic compound.
Formula unit The symbols used in equations and calculations to represent elements and compounds with giant structures.
Freezing The change of a liquid into a solid at constant temperature.
Freezing point The temperature at which a liquid turns to a solid. Latent heat is released to the surroundings during this process, as bonds between the particles in the solid are formed.
Gas A state of matter in which there are little attractive forces operating between the particles. The individual particles move at high speed and collide with each other and the walls of the container.
Gas laws The various laws that describe the physical behaviour of gases.
Gay–Lussac’s law of combining gas volumes The volume of gases taking part in a chemical reaction show simple whole number ratios to one another when measured at the same temperature and pressure.
Graham’s law of diffusion Gases diffuse at a rate that is inversely proportional to the square root of their density or molar mass at constant temperature and pressure. The result follows from the relation between kinetic energy and temperature. Two different gases at the same temperature have the same average kinetic:
½mxvx2 = ½mYvY2
vx2 / vY2 = mY/ mx
vx/ vY = √ mY/ √mx
vxµ√ 1/ √mx
Gravimetric analysis A method of quantitative analysis for finding the composition and formulae of compounds based on accurate weighing of reactants and products.
Heating curve A plot of temperature against time for a substance when heated at a constant rate.
Hydrate A salt associated with a definite number of molecules of water.
Hydrated ion An ion which has a definite number of water molecules associated with it.
Hydration A process where an unsaturated molecule adds a water molecule or water molecules interact with ions in aqueous solution.
Ideal gas An ideal gas is a hypothetical substance that consists of molecules or atoms that occupy almost no space and have no intermolecular forces. All collisions between the molecules or atoms are perfectly elastic. An ideal gas obeys the gas laws exactly under all conditions.
Ideal gas constant (R) The ideal gas constant (R) is the constant that appears in the ideal gas equation (PV = nRT).
Ideal gas equation This is an equation relating to the temperature, pressure, volume and amount of an ideal gas. It is frequently used in experimental work to determine the molar masses of gases.
Kelvin scale A temperature scale starting from absolute zero (–273°C) using degrees the same size or magnitude as degrees Celsius. The temperature in kelvin is proportional to the average kinetic energy of the particles.
Kinetic energy (of particles in a gas) The energy due to the motion; it depends on the mass of the gas particle (atom or molecule) and the square of their speed. Kinetic energy = ½mv2.
Kinetic theory This explains the physical properties of solids, liquids and gases in terms of the movement of particles (atoms, ions or molecules). The theory also accounts for changes of state.
Lattice A regular, repeating three-dimensional arrangement of atoms, molecules or ions within a crystal.
Law of conservation of mass Mass is not lost or gained during a chemical reaction. The total mass of the reactants equals the total mass of the products.
Limiting reactant This determines the theoretical yield of product. The reactant that is completely consumed, or used up, when the reaction goes to completion. The other reagents are in excess.
Liquid A state of matter in which particles are loosely attracted by intermolecular forces. A liquid takes up the shape of the walls of its container and has a fixed volume. The particles have an irregular arrangement.
Melting The change of a solid into a liquid at constant temperature. Latent heat is needed to break the bonds between the particles in the solid state.
Melting point The temperature at which a solid is converted to a liquid. It can be used to test the identity or the purity of a sample.
Molar gas volume One mole of an ideal gas occupies 22.4 dm3 at 0ºC (273 K) and one atmosphere pressure.
Molarity A measure of the concentration of a solution: the amount in moles of solute in 1 dm3 of solution. Units are mol dm–3 or sometimes simply M.
Molar mass The mass in grams of one mole of molecules or the formula units of an ionic compound. It is numerically equal to the relative molecular, atomic or formula mass of a substance in units of g mol–1.
Mole The unit of amount of a substance. One mole of a substance has a mass equal to its formula mass in grams. One mole of a substance contains 6.02 ´ 1023 (Avogadro’s constant) of atoms, ions or molecules.
Molecular formula This shows the number of atoms of each element present in a molecule.
Molecular mass The molecular mass is the sum of the atomic masses of the elements in the formula of a covalent compound.
Molecule A group of atoms held together by covalent bonds.
Particle (in kinetic theory) The term particle refers to atoms, molecules or ions.
Pascal The SI unit of pressure. One Pascal, abbreviated to Pa., is equivalent to a force of one 1 M on 1 m2.
Percentage composition The percentage by mass of each of the elements in a compound.
Percentage yield The experimental yield as a percentage of the theoretical yield:
Percentage yield = (experimental yield/ theoretical yield) ´ 100%
Phase A physically or chemically distinct part of a system. A phase is homogenous throughout and is separated from other phases by a phase boundary.
Pressure A measure of the force pressing on a surface. The pressure that a gas exerts upon its container is caused by the particles colliding with the walls of the container. Pressure is defined as force per unit area. Pressure = force / area
Pressure law The gas law stating that the pressure of a fixed mass of an ideal gas, at constant volume, is directly proportional to its absolute temperature. P µ T.
Primary standard A substance which can be used to prepare a standard solution with a precise concentration for volumetric analysis.
Product A substance produced during a chemical reaction.
Reactant A substance that is consumed during a chemical reaction.
Reacting masses The masses of elements and compounds which take part in a chemical reaction.
Real gas A real gas does not obey the gas laws and exhibits non ideal behaviour. Its molecules have a finite size and there are intermolecular forces of attraction.
Recrystallisation A technique for the purification of solid crystalline substances. The procedure is based on the significant increase of solubility of the solutes with increasing temperature.
Redox titration A titration used to determine the concentration of a solution of an oxidising agent or of a reducing agent in a redox reaction.
Relative atomic mass The average mass of an atom of an element, according to relative
abundances of its isotopes in a naturally occurring sample, on a scale where the mass of one atom of is 12 exactly.
Saturated solution A solution which contains the maximum amount of dissolved solute in a solvent at a particular temperature.
Side reactions Unwanted reactions which reduce the yield of the product being formed from the main reaction.
Solid A state of matter with particles in fixed positions and not able to move from one location to another. The particles are held in a lattice by chemical bonds or intermolecular forces.
Solubility The amount of solute required to form a saturated solution in a given volume (e.g.
1 dm3) of solvent.
Solute A solute is the solid, liquid or gas that has been dissolved to form a solution.
Solution A solution is a homogeneous mixture of a liquid (the solvent) with another substance (the solute). The solute can be solid, liquid or gas but the solvent is generally a liquid.
Solvation The process of the solute particles dissolving in a solvent as they spread out and are surrounded by solvent molecules.
Solvent A solvent is a liquid that dissolves a solute to form a solution.
Standard solution A solution with an accurately known concentration made by dissolving a known amount of a primary standard in water.
Standard state The most stable state for a specific element (in a particular allotropic form, where appropriate) under standard conditions of temperature and pressure.
State symbols Symbols used in equations to describe the physical state of the substances that are participate in a reaction. Solid (s), liquid (l), gas (g) or dissolved in water, that is aqueous, (aq).
Stoichiometry The ratio in which the elements combine in a compound or the ratio in which compounds react in a reaction.
Sublimation The direct change of state from solid to gas without melting or freezing occurring.
Temperature A measure of the average kinetic energy of the particles in a substance. The absolute temperature, measured in K, is proportional to the average kinetic energy of the particles.
Theoretical yield The theoretical yield is the mass or amount of product produced according to the chemical equation assuming 100% reaction of the limiting reagent.
Titration A chemical technique in which one solution is used to analyse another solution to find the concentration or amount of solute.
Volatility A qualitative measure of how readily a liquid or solid is vaporised upon heating or evaporation.
Water of crystallisation Water molecules that are incorporated into the crystal lattice of many inorganic salts when they crystallise from aqueous solution.
Word equation A summary of a chemical reaction using the chemical names of the reactant and products. They are not appropriate at IB level.