IB Chemistry Topic 10 Organic Chemistry notes
Summary of reaction pathways and reaction conditions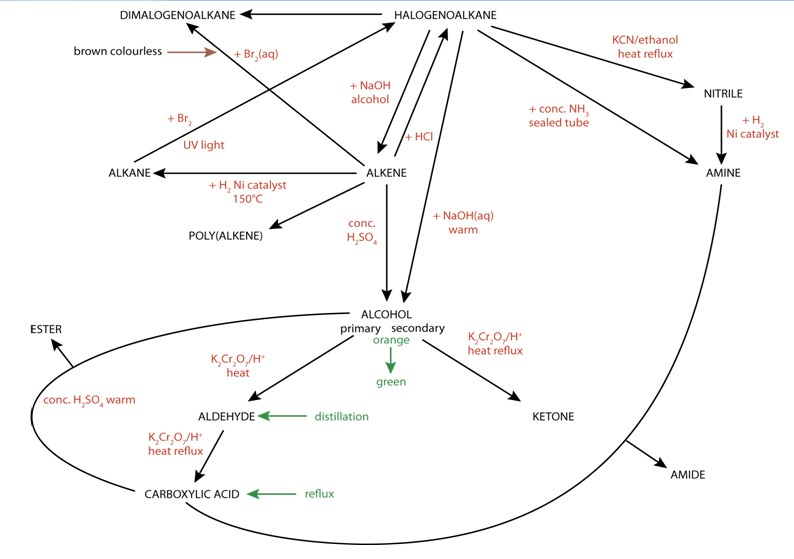
IB Chemistry topic 10 Organic Chemistry Definitions – IB Chemistry notes PDF
Addition–elimination reaction A reaction in which two molecules combine with the release of a small molecule, often water. This type of reaction is also known as condensation.
Addition reaction A reaction in which two reactants combine to form a single product. This type of reaction is typical of unsaturated compounds.
Alcohol A homologous series containing the functional group –OH. Their general formula is
CnH2n + 1 OH.
Aldehyde A homologous series containing a carbonyl group –CHO. Aldehydes are formed by the gentle oxidation of primary alcohols or by the reduction of carboxylic acids.
Alkane Saturated hydrocarbons. They have the general formula CnH2n + 2.
Alkene Unsaturated hydrocarbons. They contain a carbon–carbon double bond. They have the general formula CnH2n.
Amide A homologous series derived from carboxylic acids, with the functional group –CONH2. Secondary and tertiary amides respectively have one or two of the hydrogen atoms substituted by alkyl groups.
Amine A homologous series containing the functional group –NH2. Secondary and tertiary amines respectively have one or two of the hydrogen atoms substituted by alkyl groups.
Topic 10 Organic Chemistry – IB Chemistry notes PDF
Carbocation A species formed as an intermediate during a reaction that has a positive charge on a carbon atom. Tertiary carbocations are more stable than secondary which are more stable than primary, due to the positive inductive effect of the alkyl groups.
Carboxylic acid A homologous series containing the organic acid group –COOH.
Catenation The formation of chains of atoms in compounds. Carbon has the ability to do this to a large extent, which is one of the reasons for the vast numbers of organic compounds that exist.
Chiral A carbon atom or a molecule in which four different groups are bonded to the same carbon atom. It gives rise to optical isomers, that is two non-superimposable mirror image forms known as enantiomers.
Condensation A reaction in which two molecules combine with the release of a small molecule, often water. This type of reaction is also known as addition–elimination.
Condensed structural formula A representation of the structural formula that contains the minimum information for the sequence of atoms in the molecule to be known non-ambiguously.
Curly arrow A convention for depicting the movement of an electron pair in a reaction mechanism. The arrow is drawn from the site of the electron availability to the site of electron deficiency.
Elimination A reaction in which a small molecule is lost from a larger compound.
Empirical formula The simplest whole number ratio of the atoms in a compound.
Enantiomer One of two forms of a chiral compound, which is a mirror image (non-superimposable) of the other.
Ester Compounds which contain the group –COOR. They are the product of the reaction between carboxylic acids and alcohols.
Topic 10 Organic Chemistry – IB Chemistry notes PDF
Fossil fuels Fuels that are derived from the remains of living organisms. They include coal, oil, gas, peat, etc. When burned they produce carbon dioxide and water in exothermic reactions.
Fractional distillation The process used to separate a mixture of liquids according to their boiling points. It is used for the separation of crude oil into fractions, which have a smaller range of boiling points.
Free radical A species that contains an unpaired electron and is highly reactive. Radicals form from homolytic fission.
Functional groups An atom or small group of atoms that gives characteristic properties to the compound.
Geometric isomerism Isomers that have different spatial arrangements as the result of restricted rotation in the molecule. This arises where there is a cyclic structure or a carbon–carbon double bond.
Greenhouse gases Gases which absorb longer wave-length (infrared) radiation and so prevent the re-radiation of this from the earth, causing an increase in the temperature – global warming. The most common greenhouse gases are carbon dioxide, methane and water vapour.
Halogenoalkane Homologous series containing a halogen atom as the functional group.
Heterolytic fission When a covalent bond breaks in a reaction and both the bonded electrons go to one of the products, so giving rise to two oppositely charged ions.
Homologous series Families of organic compounds whose members can be represented by the same general formula, with successive members differing by –CH2. Members of the same homologous series show similar chemical properties and show a gradation in physical properties.
Homolytic fission When a covalent bond breaks in a reaction and each of the bonded electrons goes to one of the products, so giving rise to two free radicals.
Hydration The addition of water to a compound. For example, alkenes are hydrated to form alcohols.
Hydrocarbon A compound containing carbon and hydrogen only.
Hydrogenation The addition of hydrogen to a compound. For example, alkenes are hydrogenated to form alkanes.
Isomers Molecules which have the same molecular formula, but different structural formulas or different arrangements of the atoms in space.
Topic 10 Organic Chemistry – IB Chemistry notes PDF
IUPAC International Union of Pure and Applied Chemistry. It is best known for its system of nomenclature for organic compounds.
Ketone A homologous series containing a non-terminal carbonyl group R–CO–R. Ketones are formed by the oxidation of secondary alcohols.
Leaving group An atom or group of atoms that is displaced during a substitution or an elimination reaction.
Molecular formula The actual number of atoms present in a molecule. It is a multiple of the empirical formula.
Monomer A molecule used as the building block of a polymer. Monomers may react together by addition reactions giving rise to addition polymers; or by condensation reactions, giving rise to condensation polymers.
Nitrile A homologous series containing the functional group –CN, having a triple bond between the carbon and nitrogen atoms.
Nomenclature A precise means of naming entities so that they can be communicated non-ambiguously. Organic chemistry uses the system of IUPAC nomenclature.
Nucleophile An electron-rich species that is therefore attracted to parts of molecules that are electron deficient. Nucleophiles have a lone pair of electrons and may also have a negative charge.
Nucleophilic substitution A substitution reaction in which a nucleophile is the attacking species on a molecule, and substitutes for an atom or group in the molecule.
Optical activity The property of enantiomers to rotate plane-polarised light through equal and opposite directions.
Optical isomers Isomers that have different spatial arrangements as a result of there being four different groups tetrahedrally bonded to a single carbon atom. These isomers are non-superimposable mirror images of each other and are known as enantiomers.
Topic 10 Organic Chemistry – IB Chemistry notes PDF
Plane-polarized light Light which oscillates in a single plane, due to having been passed through a polarizer.
Polarimeter An instrument used to detect the amount and direction of rotation of plane-polarized light by a chiral compound.
Polyamide A polymer containing the amide link as the result of reaction between an acid group and an amine group in the monomers.
Topic 10 Organic Chemistry – IB Chemistry notes PDF
Polyester A polymer containing the ester link as the result of reaction between an acid group and an alcohol group in the monomers.
Polymer A long chain molecule made of repeating sub-units known as monomers.
Polythene An addition polymer of ethane with the formula for the repeating unit being – (CH2)n.
Positive inductive effect The tendency of an atom or group to push electrons away from itself. These groups, such as alkyl groups, are also known as electron-releasing groups.
Primary carbon atom A carbon atom that is attached to the functional group and also to at least two hydrogen atoms.
PVC Polyvinylchloride, the addition polymer of chloroethene. The formula for its repeating unit is –CH2CHCl–.
Racemic mixture A mixture containing equal amounts of the two enantiomers of a chiral compound. This mixture is not optically active.
Reaction pathways A series of linked reactions in which a reactant is converted in several steps into a product. The product of the first step is the reactant for the second step, etc.
Reflux condenser Apparatus used to prolong a reaction when the products may be volatile and escape before they have time to react completely. It consists of a vertical distillation column, which allows escaping vapour to be condensed and returned to the reaction vessel.
Repeating unit The smallest part of a polymer which shows how the units are linked to form the chain.
Resolution A process used to separate the enantiomers from a racemic mixture. It is often used in the pharmaceutical industry before carrying out separate trials of the two enantiomers.
Topic 10 Organic Chemistry – IB Chemistry notes PDF
Saturated A compound which contains only single bonds between carbon atoms, no carbon–carbon double or triple bonds.
Secondary carbon atom A carbon atom that is attached to the functional group and also to one hydrogen atom.
SN1 Substitution nucleophilic unimolecular. A substitution reaction in which a nucleophile attacks a molecule and the mechanism involves a single reactant in the rate-determining step. It is characteristic of the reactions between halogens and tertiary halogenoalkanes.
SN2 Substitution nucleophilic bimolecular. A substitution reaction in which a nucleophile attacks a molecule and the mechanism involves two reactants in the rate-determining step. It is characteristic of the reactions between halogens and primary halogenoalkanes.
Stereochemical formula A representation of a structural formula which shows the relative positions of atoms and groups around carbon in three dimensions.
Stereoisomerism Compounds which have the same molecular formula but atoms arranged in different spatial arrangements. There are two main types of stereoisomerism: geometric isomers and optical isomers.
Steric hindrance The effect where a chemical reaction is slowed or prevented by the presence of one or more bulky groups on a molecule, which prevent the approach of another reactant to the reaction site.
Structural formula A representation of a molecule, showing the order of attachment of its atoms.
Structural isomers Compounds which have the same molecular formula but different structural formulas – different order of attachment of the atoms.
Substitution reaction A reaction where one atom or group of atoms in a compound is replaced by a different atom or group. This type of reaction is typical of saturated compounds.
Topic 10 Organic Chemistry – IB Chemistry notes PDF
Tertiary carbon atom A carbon atom that is attached to the functional group and also to three alkyl groups. It is not bonded to any hydrogen atoms.
Unsaturated A compound that contains a double or triple bond between carbon atoms.








